“Anti-acne, Anti-inflammatory and Anti-oxidant properties
of POMEWHITE”
A placebo and randomized controlled trial
Study Conducted Under Medical Control
CLINICAL TRIAL DESIGN
01
02

Subject: 100 volunteers with moderate to severe acne was recruited and 20 are healthy volunteers assigned as a control group
03
Duration: 8 weeks study (56 Days)
September 1, 2021 – Oct 27, 2021
Test Group: volunteers with moderate to severe acne
04
Dosage: 150mg and 300mg of PomeWhite in capsule given per day to participants
PRIMARY OBJECTIVE
The primary objective of this study was to evaluate the POMEWHITE® potential for decreasing the amount of acne by increasing the probiotics of skin and controls the oil production of the skin.
SECONDARY OBJECTIVE
The secondary objectives were to evaluate POMEWHITE® food supplement for:
1. POMEWHITE® treatment could be viable option to reduce the acne lesions inflammation via increasing interferon-gamma and lowering the interlukin-4.
2. POMEWHITE® could also exert its effect for scar reduction and give brightened look to skin by anti-tyrosinase activity.
3. Removal of fine lines due to scary skin is another characteristic of POMEWHITE®.
4. POMEWHITE® could has unique ability to reduce the oxidative stress of the skin contributing to overall wellness of the skin.

ASSESSMENT OF DIFFERENT HEALTH PARAMETERS
Assessment of Skin Health and Microbiota Sampling
The primary objective of this study was to evaluate the POMEWHITE® potential for decreasing the amount of acne by increasing the probiotics of skin and controls the oil production of the skin.
The primary outcome measure was bacterial index measurement (probiotic/normal flora and acne producing bacteria colony count) and acne lesion count (total, inflammatory, and non-inflammatory) and depicted as levels of acne by expert dermatologists. For this purpose inflammation index and the total number of lesions will be recorded at every follow up.
Estimation of Anti-Oxidative Enzymes
Normally, the production of free radicals is slow and they are removed by the antioxidant enzymes existing in the cell. First line of defense from ROS include antioxidant enzymes including Superoxide dismutase (SOD) and glucose-6-phosphate dehydrogenase (G6PD).
Estimation of Superoxide Dismutase (SOD)
For the evaluation of SOD in pre-treated and post-treated groups of POMEWHITE® capsules, the harvested serum was used, and SOD estimation was done via kit according
to the manufacturer’s protocol (Sigma Aldrich). The results were measured in units/ml of serum.
Estimation of Glucose-6-Phosphate Dehydrogenase (G6PD)
G6PD in pre-treated and post-treated groups of POMEWHITE® capsules was estimated by using the blood of volunteers taken in EDTA containing tubes, and G6PD estimation was done via kit according to the manufacturer’s protocol (Sigma Aldrich). The test results were measured as units per gram of hemoglobin.
Enzyme Linked Immuno-Sorbant Assay (ELISA)
Solid phase sandwich ELISA was implemented for IL-4 (inflammation) and INF-γ (inflammation) by the previously reported Wajid et al, (Wajid, Naseem et al. 2015) method with slight modifications.
Lipase Activity
Lipase activity was done by using the serum lipase estimation kit. This test was done as more lipase in serum can cause more free fatty acids presence in the blood, thus promoting acne like problems.
Tyrosinase Activity
Tyrosinase activity was calculated by method of Chan, Kim et al. with some modifications (Chan, Kim et al. 2011). This test evaluate the potential of POMEWHITE® for skin brightening. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. So this test evaluate the melanin production.
CLINICAL STUDY RESULTS
POMEWHITE® Food Supplement Decreases Skin Acne
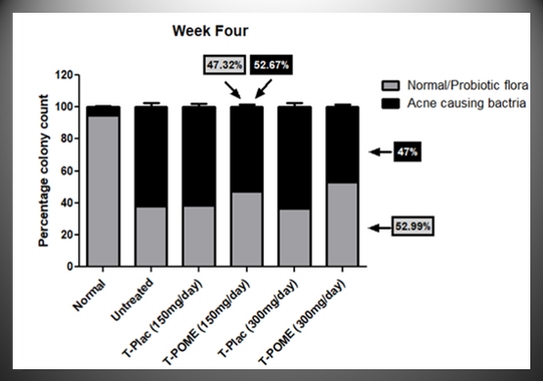
Skin Microbiota Assessments, colony count of skin microbiota was estimated by using swabbing and culturing of bacteria to evaluate the POMEWHITE® effects for the reduction of acne producing bacteria. Culturing was done from week zero, four and eight samples and results were obtained after 48 hours of culturing.
The graph reveals that at week zero number of acne producing bacteria colonies was higher in volunteers as compared to normal controls.
At week four post POMEWHITE® treatment (Figure 2b), the acne producing bacteria started decreasing in number and probiotic colonies started increasing in acne patients.
At eight weeks at 300mg/day dose resulted in a significant decline in acne producing bacteria colonies compared to the untreated group, where a significant increase in probiotic/normal flora was observed.

Values were expressed as ±SEM. (p<0.05 consider as significant) (* shows the significant difference between treated groups and untreated control of same week) (α indicates the significance of 150mg/day groups of 4 and 8 weeks vs. zero week) (β indicates the significance of 300mg/day groups of 4 and 8 weeks vs. zero week).
The graph (figure 2e) reveals that eight weeks intake of POMEWHITE® at 300mg/day dose results in a significant improvement in overall wellbeing of the skin as compared to the untreated group, where an unsatisfied index is high among the participants. Additionally, 300mg/day dosage of POMEWHITE® was more effective in decreasing the acne levels and improving skin than 150mg/day consumption of supplement for 8 weeks. Relative percentage analysis (figure 2f) further strengthens this fact that T- POME (300mg/day, 8weeks) group displays strong satisfactory index to participants.
Before and After Photos
Morphological Quantification of Skin Acne
Evaluation of acne reduction of skin in pre-treatment and post-treatment groups of POMEWHITE® was done by quantification of pre-treatment and post-treatment face images with the help of Image J software. Figure 2g has shown a visible decrease in acne levels of skin after 8-weeks intake of POMEWHITE® at a dose of 300mg/day for 8 weeks.
Figure 2g: A) Skin morphology at day zero treatment of POMEWHITE®. B) Skin morphology at day 56 (week 8) after POMEWHITE® treatment (300mg/day). (Red color corresponds to the skin redness due to acne lesions or scars)(Images are quantified by image j software)

Evaluation of acne reduction of skin in pre-treatment and post-treatment groups of POMEWHITE® was done by quantification of pre-treatment and post-treatment face images with the help of Image J software. Figure 2h has shown a visible decrease in acne levels of skin after 8-weeks intake of POMEWHITE® at a dose of 300mg/day for 8 weeks.
Figure 2h: A) Skin morphology at day zero treatment of POMEWHITE®. B) Skin morphology at day 56 (week 8) after POMEWHITE® treatment (300mg/day). (Red color corresponds to the skin redness due to acne lesions or scars)(Images are quantified by image j software)

Numerical Scale of acne levels were determined in all subjects at weeks zero, 4, and 8. j) Relative percentage analysis of acne levels among different experimental groups. The normal group indicates healthy persons with skin type 1 and 2, the untreated group includes persons with Skin type 3-5 with no treatment, T-Plac (150mg/day) group includes persons skin type 3-5 treated with rice powder 150mg/day, T- POME (150mg/day) includes participants skin type 3-5 treated with POMEWHITE® 150mg/day, T-Plac (300mg/day) represents participants skin type 3-5 treated with rice powder 300mg/day and T- POME (300mg/day) includes participants skin type 3-5 treated with POMEWHITE® 300mg/day.
Effect of POMEWHITE® Food Supplement On Skin Stress
The graphs in figure 3a and b show that in the untreated groups, SOD levels were significantly increased. Additionally, the graph also indicates that POMEWHITE® at a dose of 300mg/day post-eight-weeks intake reduces the SOD levels to a considerable extent compared to the 150mg/day dose of POMEWHITE®.
The graphs in figure 3c and 3d show that in the untreated groups, G6PD levels were significantly decreased. Additionally, the graph also indicates that POMEWHITE® at a dose of 300mg/day post-eight-weeks intake augments the G6PD levels to a considerable extent compared to the 150mg/day dose
Effect on Inhibition of Tyrosinase Activity

Figure 4 has shown a visible increase in tyrosinase inhibition that further resulted in lightened the skin tone after 8-weeks intake of POMEWHITE® at a dose of 300mg/day. The graphs in figure 3c also indicates that, at 8th-week post POMEWHITE® oral intake 300mg/day, an apparent increase in percentage activity of inhibition of tyrosinase was observed as compared to placebo and untreated groups.
Effect on reduction of fine lines and wrinkles
Figure a:graph indicates that a seeming reduction in the face fine lines and wrinkles at 8th weeks after POMEWHITE® (300mg/day) oral consumption was observed in comparison with untreated group.
Figure b: indicates the relative percentage analysis of reduction in fine lines and wrinkles in POMEWHITE® post treated group (300mg/day, 8week) as compared to other groups.
POMEWHITE® food supplement lessens skin inflammation
Figure a: reveals that eight weeks intake of POMEWHITE® at 300mg/day dose results in a significant decline in IL-4 expression levels compared to the untreated group, where an upsurge in levels can be seen.
Figure b: Further strengthens this fact that IL-4 levels in T- POME (300mg/day) group are similar to that of normal group levels. No alteration was seen in IL-4 expression in vehicle rice powder group participants.
Figure c: Indicates that 300mg/day dosage of POMEWHITE® for 8 weeks was more effective in improving IFN-γ as compared to POMEWHITE® at 150mg/day dosage. Whereas low levels of IFN-γ were observed in the untreated group.
Figure d: Reveals that the levels of IFN-γ in T- POME (300mg/day) group are non-significantly different from the normal groups, while rice powder intake has no impact on IFN-γ levels.
CONCLUSION
The unique natural formula of POMEWHITE® augments the increase of probiotics levels in acne-damaged skin that not only eliminates acne but also contributes to repair the damaged skin and enhances the skin tone.
POMEWHITE® further minimizes the acne by decreasing lipase activity. POMEWHITE® treatment group expresses low levels ROS and inflammation making POMEWHITE® a potential option for treatment of inflammation due to acne-damage and improve the skin health.
POMEWHITE
Anti-Acne, Anti-Inflammatory and Anti-Oxidant Properties